

Infusion Pumps: Pump Up the Party
What is an infusion pump?
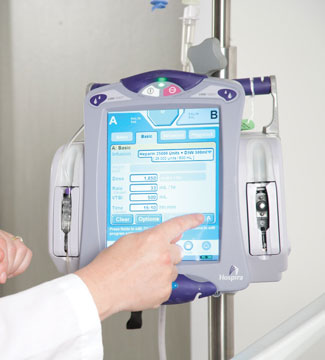
An infusion pump is a medical device that delivers nutrients and medications in the form of fluids into a patient’s body in controlled amounts. These devices can be used in a variety of clinical settings such as hospitals, nursing homes, and personal homes, delivering steady and precise amounts of nutrients or medications including insulin, or other hormones, antibiotics, chemotherapy drugs, and pain relievers. Sometimes, implementing fluids into the body can be expensive, unreliable, and inaccurate if done manually, but by being able to control the rate, duration, and amount of fluid that is delivered through the software interface, patients feel at ease, having the logistics under control, and knowing they are well taken care of.
Different kinds of infusion pumps
A number of styles of infusion pumps allow for different levels of mobility and comfort, working best with the patient’s health needs. Ambulatory infusion pumps, for instance, are designed with mobility in mind, formulating in a portable or wearable fashion. Other infusion pumps, such as large volume ones include the patient controlled analgesia (PCA), elastomeric, syringe, enteral, and insulin pumps. Each pump may have a different purpose, and work differently, according to the patient’s respective condition.
The enteral infusion pump, for example, is best for delivering liquid nutrients and medications to the digestive tract, while PCA infusion pumps are used mainly to deliver pain medication. PCA pumps allow patients to manage the medication amount as needed, and insulin infusion pumps are used most often to inject insulin into patients with diabetes. This device is often used at home. Electrical and mechanical pumps also serve different functions regarding fluid control. With syringe infusion pumps, for instance, fluid is held inside the syringe reservoir, and a moveable piston controls fluid delivery. Elastomeric infusion pumps hold fluid in elastic balloon reservoirs, allowing the pressure from the walls of the balloon to deliver the fluid. Peristaltic pumps, on the other hand, include a set of rollers that attach and pinch tubing, allowing fluid to push forward. Some infusion pumps are even able to deliver fluids from several reservoirs.
Are they safe?
Infusion pumps, on top of regulating the levels of infusion, often come with safety features, including alerts and alarms that are programmed to activate in the face of an issue regarding the patient’s health or safety. Smart pumps, a new line of infusion pumps, are designed to notify users when the patient is at risk of a drug interaction,
or when the system’s parameters are set outside of the safety limits. However, health care providers must use sound and professional judgment. Drug libraries, or error reduction software, allows healthcare providers to program and calculate appropriate dose and delivery rates for patients. If each step of the process is done properly, I.V medication errors should not exist.
However, according to the FDA, between 2005 and 2009, around 56,000 reports of problems occurred in regard to infusion pumps. During this time, manufacturers recalled 87 infusion pumps to rectify any unresolved issues. 70 of the recalls were categorized as Class II, suggesting that the health consequences were not too serious and the devices caused temporary or medically reversible health problems. 14 of the recalls were designated as Class I, meaning there was high chance that the device would result in severe health consequences or death.
These reports have not been isolated to a specific manufacturer, style of infusion pump, or the environment in which they were used; rather, many of the reported problems point to malfunctions within the device design and engineering, most commonly software defects, user interface issues, and mechanical or electrical failures. A software malfunction might include a pre-programmed alarm not activating when necessary, or other alerts sounding when there is in fact no problem at all. In other cases, software errors have caused over-infusion or under-infusion. Interface issues have been another cause for problems, as the on screen information at times may not have made data clear, such as units of measurement, confusing, for example, pounds and kilograms, which could have lead to inappropriate patient dosing. Problems might also occur due to mechanical or electrical malfunctions. Pump housings might cause some damage, early battery failures, and or pump fires and sparks that might lead to inaccurate fluid administration.
In 2010, the FDA launched the Infusion Pump Improvement Initiative to raise awareness and make certain that infusion pumps were operating and being operated properly by healthcare providers and patients in order for accurate and beneficial results.
Manufacturers
The first order of business for the FDA was to make certain that manufacturers of infusion pumps included more design and engineering information before the devices were assembled and used, so any malfunctions or issues were able to be spotted preemptively, before their use. This information has also become required in the form of a draft guidance document, which must provide detailed information for the FDA during premarket review, which includes the steps the manufacturer has taken to test the stages of the product’s life cycle, to optimize use, design, servicing, maintenance, and manufacture.
Device Improvements
By taking into account static analysis of software code, the FDA is further implementing content productivity and safety, understanding better how to identify programming errors. With constant research and attention to manufacturing details, infusion pumps are being maintained to the highest standards.
User awareness
User awareness is crucial, as patients and healthcare professionals alike must understand the functions and operations of this comprehensive device. With information online, users are encouraged to research and do their homework before operating and being operated on.
References:
Cantrell, Susan. “August 2012 – Infection Prevention.” August 2012 – Infection Prevention. N.p., Aug. 2012. Web. 28 Jan. 2016. <http://www.hpnonline.com/inside/2012-08/1208-IP-Pumps.html>.
“U.S. Food and Drug Administration.” White Paper: Infusion Pump Improvement Initiative. N.p., n.d. Web. 28 Jan. 2016. <http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/GeneralHospitalDevicesandSupplies/InfusionPumps/ucm205424.htm#types>.



One thought on “Infusion Pumps: Pump Up the Party”
Comments are closed.