-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

3M 1684 - TEGADERM, DSG, 2 3/8 X 2 3/4 DIAMOND, 400/CS
3M Tegaderm Diamond Pattern Film Dressing with Comfort Adhesive Technology
Increased Breathability for Secure Hold
3M Tegaderm Diamond Pattern Film Dressing consists of a thin film backing with a non-latex, hypoallergenic adhesive. The dressing is breathable, allowing good oxygen and moisture vapor exchange. It is waterproof and impermeable to liquids, bacteria and viruses*. An intact dressing protects the site from outside contamination.
- * In vitro testing shows that the film of Tegaderm Diamond Pattern Film Dressings provides a viral barrier from viruses 27 nm in diameter or larger while the
dressing remains intact without leakage.
Indications for Use
3M Tegaderm Diamond Pattern Film Dressing can be used to cover and protect catheter insertion sites and minor wounds. It can also be used as a secondary dressing, as a protective cover over at-risk skin, to secure devices to the skin and as a protective eye covering. Do not use the dressing as a replacement for sutures and other primary wound closure methods.
Suggested Applications
- To cover and protect I.V. catheter sites
- Minor wounds
- Skin protection
- Clean, closed surgical incisions
- Abrasions, skin tears, blisters
- Skin graft donor sites
- Protective eyelid coverings
- Post tattoo application and removal
Precautions
- Stop any bleeding at the site before applying the dressing.
- Do not stretch the dressing during application as tension can cause skin trauma.
- Make sure the skin is clean, free of soap residue and lotion and allowed to dry thoroughly before applying the dressing to prevent skin irritation and to ensure good adhesion.
- The dressing may be used on an infected site, only when under the care of a health care professional.
- Antimicrobial ointments containing polyethylene glycols may compromise the strength of the Tegaderm Diamond Pattern Film Dressing.
- Tegaderm Diamond Pattern Film Dressing should not be re-sterilized by gamma, E-beam or steam methods.
Composition/Information on Ingredients
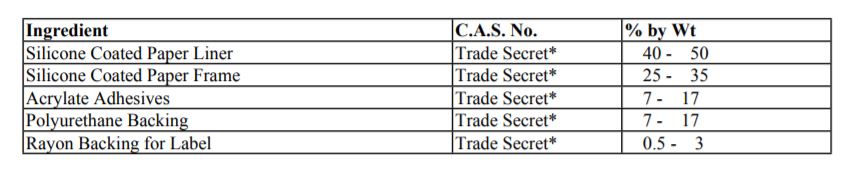
*The specific chemical identity and/or exact percentage (concentration) of this composition has been withheld as a trade secret.

Helping You Deliver Exceptional Patient Care
Every day, we're inspired by all you do. Were committed to helping you with people, products and ideas that simplify and improve the standard of I.V. site care. Thats why we leveraged our customer insights and proven leadership to help you provide the comfort and protection your patients deserve.
Provides secure hold
Reinforced border, deep notch, and tape strips work together to enlist the entire dressing in securement. Innovative adhesives hold strongly, manage moisture, and release easily. Designed to maintain securement by preventing edge lift, flexing with patient movement, and managing moisture.
Easy to apply and remove
Designed to promote consistent application. Frame delivery makes placement accurate and easy.
Excellent value
Can potentially reduce the number of dressing changes and restarts. May be worn for up to 7 days on a PIV catheter. Provides up to 7 days of wear time for CVCs**
Supports infection prevention goals
A waterproof film coating on all pieces provides a barrier to external contaminants including liquids, bacteria and viruses*. Allows continuous monitoring.
Application & Removal Guide


Specifications

Disposal Considerations
Dispose of contents/container in accordance with the local/regional/national/international regulations.

