-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

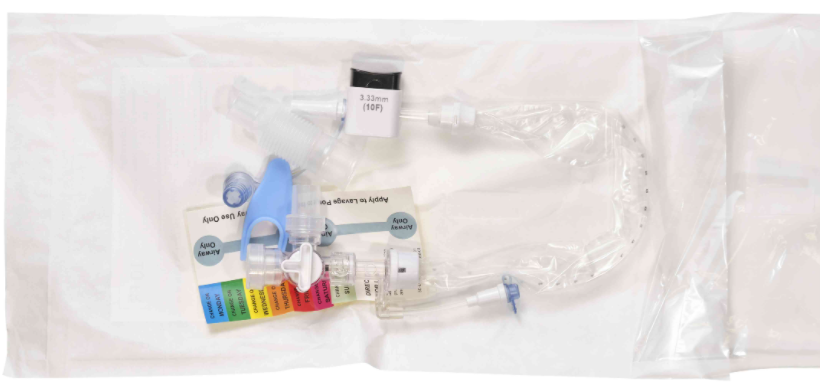
Avanos Medical 01-EC-459-1401 - Liberator CS, Closed Suction System with Manifold, 14 FR, 10 EA/CS
Avanos 01-EC-459-1401 Liberator CS, Closed Suction System with manifold
The AVANOS Liberator CS, Closed Suction System is a 72 hour closed suction catheter that is compatible with the Liberator HCS, ET Tube Clearing System and manifold. Manifold stopcock feature prevents lung de-recruitment during catheter exchange and Bronch / BAL Adapter use.
Avanos 01-EC-459-1401 Liberator CS, Closed Suction System with manifold Features
- Sterile
- 72-hour use
- Isolated rinsing chamber for cleaning the catheter without washing saline down the ETT
- Versatile manifold with optional BAL bronchoscopy adapter
- Isolated rinsing chamber for cleaning the catheter without washing saline down the ETT
- Radio opaque catheter
- Versatile manifold with optional BAL bronchoscopy adapter
Introducing BALLARD Liberator, ET Tube Clearing System
Clears the ET tube to help liberate your patients from the vent sooner
The Challenge:
Many patients capable of being weaned from the ventilator may fail their spontaneous breathing trial (SBT) because of increased work of breathing from a narrowed lumen. Routine endotracheal suctioning is unable to remove adherent secretions and biofilm from the walls of the ET tube, resulting in:
- A narrowed airway
- Increased airway resistance
- Increased work of breathing
- Delayed extubation
The Win:
BALLARD Liberator restores performance of the endotracheal tube, giving patients an improved opportunity to pass their SBT.
- Clears the ETT of tenacious secretions and biofilm
- Reduces airway resistance and work of breathing
- Restores the ETT to optimal weaning conditions
- Gives patient an improved opportunity to pass their SBT
Clear tubes reduce resistance allowing patients to liberate from the vent sooner

Study objective: Compare the effectiveness of removing adherent endotracheal tube secretions prior to weaning trials, compared to the effectiveness of routine suctioning alone prior to weaning trials. Design: Three-year retrospective study of all adult patients, age 18 or older, admitted to the Intensive Care Unit who were on the ventilator greater than 24 hours. 583 cases were reviewed on year one, 516 on year two, and 662 on year three. Results: The removal of adherent endotracheal tube secretions prior to weaning trials decreased average ventilator days by up to a day
Unique wiping technology makes it possible

- Can be used for 72 hrs
- Easy to use handle with audible click
- Simplified sizing: 7.0-7.5 mm or 8.0-8.5 mm models
- Silicone construction for soft suction catheter
- Isolated rinsing chamber for cleaning catheter without washing saline down the ETT
- Radio opaque catheter
- Versatile manifold with optional BAL bronchoscopy adapter
Avanos Liberator CS, Closed Suction System with manifold 01-EC-459-1401 Specifications
| Name | Value |
| Diameter (Fr) | 12 |
| Product Configuration | Initial Placement Kit |
| Sterile | Yes |
| Sterilization Method | Ethylene Oxide |
| Depth Marks in Centimeters | Yes |
| Catheter Length (cm) | 59.5.0 |
| Catheter Material | Silicone |
| Manifold Type | Double Swivel Elbow |
Avanos Liberator CS, Closed Suction System with manifold 01-EC-459-1401 Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as ""Not made with natural rubber latex"": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |

Avanos Medical #01-EC-459-1405, Liberator CS, Closed Suction System without Manifold, 14 FR, 10 EA/CS
$251.43 per CASE

Avanos Medical #01-EC-459-1201, Liberator CS, Closed Suction System with Manifold, 12 FR, 10 EA/CS
$334.19 per CASE

Avanos Medical #01-EC-459-1205, Liberator CS, Closed Suction System without Manifold, 12 FR, 10 EA/CS
$351.43 per CASE

Avanos Medical #01-EC-254-1201, Liberator HCS, Hybrid Closed Suction System with Manifold, 7.0-7.5 ETT, 12 FR, 10 EA/CS
$1,169.64 per CASE