-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

B Braun 4252519-02 - Introcan Safety IV Catheter 22 Gauge x 1 Inch, FEP, Straight for Secure and Safe IV Access, 50/BX, 4 BX/CS (Rx)
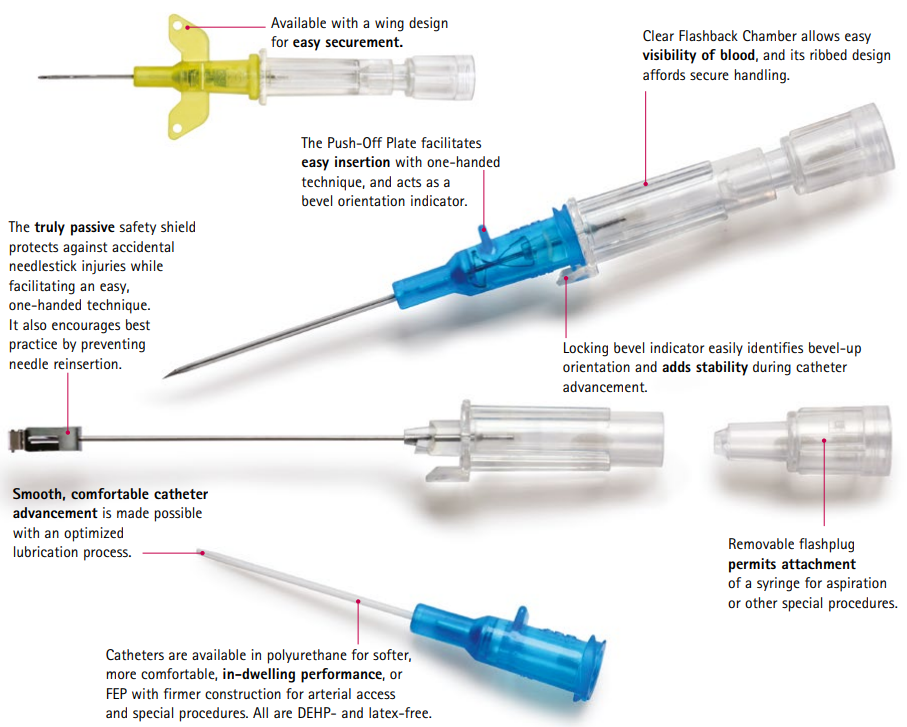
The B Braun 4252519-02 Introcan Safety IV Catheter is a 22-gauge, 1-inch intravenous (IV) catheter designed for secure, safe, and reliable IV access. Made from FEP (Fluorinated Ethylene Propylene), this catheter offers flexibility and durability while maintaining a straight, easy-to-insert design. The Introcan Safety IV Catheter features a passive safety mechanism that automatically activates upon removal, helping to prevent accidental needle-stick injuries and enhancing the safety of healthcare providers and patients alike.
Ideal for various clinical applications, this catheter is suited for hospitals, emergency care, and outpatient settings where safety and reliable IV access are paramount.
Key Benefits
- 22 Gauge, 1-Inch Length: Offers precise and suitable access for IV therapy in both adult and pediatric patients, ideal for general infusion and medication administration.
- FEP Material: Provides flexibility, durability, and smooth insertion while maintaining catheter patency and minimizing kinking.
- Passive Safety Mechanism: The needle safety guard automatically engages upon removal, reducing the risk of accidental needle sticks and increasing safety for healthcare providers.
- Straight Design: Allows for easy and secure insertion, ensuring reliable access to veins for IV therapy.
- Latex-Free: Safe for patients with latex sensitivities, reducing the risk of allergic reactions.
The B Braun Introcan Safety IV Catheter is designed with both patient and provider safety in mind, offering a secure, efficient, and sterile solution for IV access in diverse medical environments.
Compatibility and Applications
- Compatibility: Compatible with standard IV administration sets and accessories, making it versatile for various infusion therapies.
- Applications: Suitable for IV therapy, medication administration, and blood sampling in hospitals, emergency departments, outpatient clinics, and other healthcare facilities. The safety feature makes it particularly beneficial in high-risk areas where infection control and injury prevention are essential.
Usage Guidelines
To use the B Braun 4252519-02 Introcan Safety IV Catheter:
- Prepare the insertion site and insert the catheter, following aseptic techniques.
- Once the catheter is in place, retract the needle, which will activate the passive safety mechanism.
- Secure the catheter and connect to the IV line or other infusion setup as required.
- Monitor the site for proper flow and patient comfort.
- Dispose of the needle component according to facility protocols to ensure sterility and safety.
For detailed usage instructions, consult the product guide included with the catheter or contact B Braun customer support.
Certifications, Safety Standards, and Regulatory Approvals
The B Braun Introcan Safety IV Catheter meets stringent safety and quality standards:
- FDA 510(k) Cleared: Approved for medical use in the United States, ensuring compliance with safety and performance standards.
- ISO 13485 Certified: Manufactured under a certified quality management system, ensuring consistent product quality and reliability.
- Latex-Free: Safe for patients sensitive to latex, reducing the risk of allergic reactions.
These certifications ensure that the B Braun Introcan Safety IV Catheter meets the highest standards for safety, quality, and reliability in clinical environments.
Comparison with Competitor Products
The B Braun Introcan Safety IV Catheter offers a unique passive safety mechanism that distinguishes it from many competitor products, which may require manual activation of the safety feature. This automatic safety activation enhances healthcare provider safety and minimizes risks associated with accidental needle-stick injuries. The catheters FEP material and durable design also make it a preferred choice for reliable IV access in various clinical settings.
Typical User Profile and Application Scenarios
- Healthcare Professionals: Nurses, doctors, and clinical staff administering IV therapy in hospitals, outpatient clinics, and emergency care settings.
- Appropriate Scenarios: The Introcan Safety IV Catheter is ideal for patients needing continuous or intermittent IV therapy, blood sampling, or medication administration. Its safety feature makes it particularly valuable in busy or high-risk environments where needle-stick prevention is a priority.
Best Practices for Use and Maintenance
- Ensure the catheter packaging is intact and sterile before use.
- Follow aseptic techniques for insertion and securing the catheter.
- Monitor the IV site for signs of infiltration or discomfort.
- Dispose of the needle component according to facility protocols to prevent contamination and ensure safety.
Regulatory and Safety Information
The B Braun Introcan Safety IV Catheter complies with all relevant FDA and ISO safety regulations, ensuring safe and effective use in clinical settings. Its latex-free design ensures compatibility with a wide range of patients, including those with sensitivities to these materials.
How to Order
To purchase the B Braun 4252519-02 Introcan Safety IV Catheter (22 Ga. x 1 in., FEP, Straight), visit the CIA Medical website, add the product to your cart, and complete the checkout process. For bulk orders or inquiries, contact CIA Medical customer service at (312) 275-5850 or submit a request for a quote using the contact form on the product page.
This safety IV catheter provides healthcare professionals with a secure, sterile, and efficient solution for IV therapy, ensuring optimal patient care and enhanced provider safety across various medical settings.

B Braun #4252519-02, Introcan Safety IV Catheter 22 Gauge x 1 Inch, FEP, Straight for Secure and Safe IV Access, 200/CS
$582.40 per CASE

B Braun #4252519-02, Introcan Safety IV Catheter 22 Gauge x 1 Inch, FEP, Straight for Secure and Safe IV Access, 50/BOX
$175.00 per BOX

B Braun #4252519-02, Introcan Safety IV Catheter 22 Gauge x 1 Inch, FEP, Straight for Secure and Safe IV Access, 50/BX, 4 BX/CS (Rx)
$604.00 per CASE

B Braun #4252519-02, Introcan Safety IV Catheter 22 Ga. x 1 in., FEP, Straight, 200/CS
$2.68 per EACH

