-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

B Braun L7511 - Lactated Ringers Injection Solution, 5% Dextrose, 500 mL Bag, 24/cs
Lactated Ringers Injection Solution, 5% Dextrose, 500 mL Bag, 24/cs
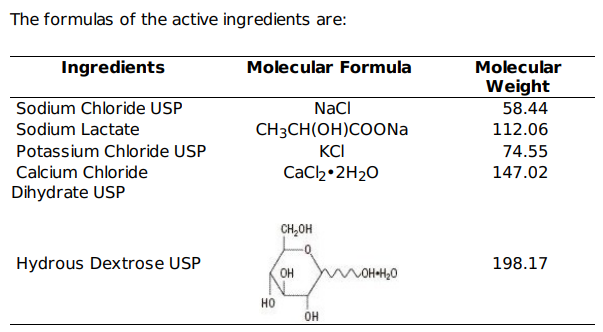
5% Dextrose in Lactated Ringer's Injection provides electrolytes and calories, and is a source of water for hydration. It is capable of inducing diuresis depending on the clinical condition of the patient. This solution also contains lactate which produces a metabolic alkalinizing effect. Sodium, the major cation of the extracellular fluid, functions primarily in the control of water distribution, fluid balance and osmotic pressure of body fluids. Sodium is also associated with chloride and bicarbonate in the regulation of the acid-base equilibrium of body fluid. Potassium, the principal cation of intracellular fluid, participates in carbohydrate utilization and protein synthesis, and is critical in the regulation of nerve conduction and muscle contraction, particularly in the heart.
Chloride, the major extracellular anion, closely follows the metabolism of sodium, and changes in the acid-base balance of the body are reflected by changes in the chloride concentration. Calcium, an important cation, provides the framework of bones and teeth in the form of calcium phosphate and calcium carbonate. In the ionized form, calcium is essential for the functional mechanism of the clotting of blood, normal cardiac function, and regulation of neuromuscular irritability. Sodium lactate is a racemic salt containing both the levo form, which is oxidized by the liver to bicarbonate, and the dextro form, which is converted to glycogen.
Lactate is slowly metabolized to carbon dioxide and water, accepting one hydrogen ion and resulting in the formation of bicarbonate for the lactate consumed. These reactions depend on oxidative cellular activity. Dextrose provides a source of calories. Dextrose is readily metabolized, may decrease losses of body protein and nitrogen, promotes glycogen deposition and decreases or prevents ketosis if sufficient doses are provided.
Lactated Ringers Injection Solution, 5% Dextrose B Braun L7511 Contraindications
The use of 5% Dextrose in Lactated Ringers Injection is contraindicated in neonates (28 days of age or younger) receiving concomitant treatment with ceftriaxone, even if separate infusion lines are used, due to the risk of fatal ceftriaxone-calcium salt precipitation in the neonates bloodstream. This solution is contraindicated where the administration of sodium, potassium, calcium, chloride or lactate could be clinically detrimental. Lactate administration is contraindicated in severe metabolic acidosis or alkalosis, and in severe liver disease or anoxic states which affect lactate metabolism. Solutions containing dextrose may be contraindicated in patients with hypersensitivity to corn products.
5% Dextrose B Braun L7511 Warnings
Precipitation of ceftriaxone-calcium can occur when ceftriaxone is mixed with calcium containing solutions, such as 5% Dextrose in Lactated Ringers Injection, in the same intravenous administration line. Do not administer ceftriaxone simultaneously with 5% Dextrose in Lactated Ringers Injection via a Y-site. Deaths have occurred in neonates (28 days of age or younger) who received concomitant intravenous calcium-containing solutions with ceftriaxone resulting from calcium-ceftriaxone precipitates in the lungs and kidneys, even when separate infusion lines were used. 5% Dextrose in Lactated Ringers Injection is contraindicated in neonates receiving ceftriaxone.
However, in patients other than neonates, ceftriaxone and 5% Dextrose in Lactated Ringers Injection may be administered sequentially if the infusion lines are thoroughly flushed between infusions with a compatible fluid. Solutions containing lactate are not for use in the treatment of lactic acidosis. Solutions containing lactate should be used with great care in patients with metabolic or respiratory alkalosis, and in those conditions in which there is an increased level or an impaired utilization of lactate, such as severe hepatic insufficiency. The administration of intravenous solutions can cause fluid and/or solute overload resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema.

The risk of dilutional states is inversely proportional to the electrolyte concentration. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentration. Solutions containing sodium ions should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency, and in clinical states in which there is sodium retention with edema. Solutions containing potassium ions should be used with great care, if at all, in patients with hyperkalemia, severe renal failure, and in conditions in which potassium ions retention is present. In patients with diminished renal function, administration of solutions containing sodium or potassium ions may result in sodium or potassium retention. Solutions containing calcium ions should not be administered through the same administration set as blood because of the likelihood of coagulation.
Lactated Ringers Injection Solution by B. Braun Precautions
This solution should be used with care in patients with hypervolemia, renal insufficiency, urinary tract obstruction, or impending or frank cardiac decompensation. Extraordinary electrolytes losses such as may occur during protracted nasogastric suction, vomiting, diarrhea or gastrointestinal fistula drainage may necessitate additional electrolyte supplementation. Additional essential electrolytes, minerals and vitamins should be supplied as needed. Sodium-containing solutions should be administered with caution to patients receiving corticosteroids or corticotropin, or to other salt-retaining patients.
Care should be exercised in administering solutions containing sodium or potassium to patients with renal or cardiovascular insufficiency, with or without congestive heart failure, particularly if they are postoperative or elderly. The osmolarity of 5% Dextrose in Lactated Ringers Injection is 530 mOsmol/liter (calc). Administration of substantially hypertonic solutions may cause venous irritation, including phlebitis. Solutions containing calcium should be used with caution in the presence of cardiac disease, particularly when accompanied by renal disease.
Parenteral calcium should be administered with extreme caution to patients receiving digitalis preparations. Solutions containing lactate should be used with caution. Excess administration may result in metabolic alkalosis. The conversion of lactate to bicarbonate is markedly delayed in the presence of tissue anoxia and reduced capacity of the liver to metabolize lactate. This may occur under conditions such as metabolic acidosis associated with circulatory insufficiency, extracorporeal circulation, hypothermia, glycogen storage disease, liver dysfunction, respiratory alkalosis, shock or cardiac decompensation. Solutions containing dextrose should be used with caution in patients with overt or known subclinical diabetes mellitus, or carbohydrate intolerance for any reason.
To minimize the risk of possible incompatibilities arising from mixing this solution with other additives that may be prescribed, the final infusate should be inspected for cloudiness or precipitation immediately after mixing, prior to administration, and periodically during administration. Do not connect flexible plastic containers in series in order to avoid air embolism due to possible residual air contained in the primary container. If administration is controlled by a pumping device, care must be taken to discontinue pumping action before the container runs dry or air embolism may result. If administration is not controlled by a pumping device, refrain from applying excessive pressure (greater than 300mmHg) causing distortion to the container such as wringing or twisting. Such handling could result in breakage of the container.
This solution is intended for intravenous administration using sterile equipment. It is recommended that intravenous administration apparatus be replaced at least once every 24 hours. Use only if solution is clear and container and seals are intact.
B. Braun L7511 Excel IV Container Feature
Excel IV containers by B. Braun are biologically inert and not made with PVC or DEHP, therefore these containers minimize patient exposure to the toxic DEHP plasticizer compared to using PVC containers that contain DEHP.

Manufacturer: L7511 B. Braun
Application: Caloric Agent
Container Type: Flexible Bag
Dosage Form: IV Solution
Generic Drug Name: Dextrose / Lactated Ringer's Solution
Strength: 5%
Type: Intravenous
Volume: 500 mL
EXCEL Flexible Plastic Bag
MADE IN USA



