-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Baxter 2B0164X - DEXTROSE INJECT USP 10%
10% Dextrose Injection, USP in VIAFLEX Plastic Container. VIAFLEX Plastic Container, 1000mL
10% Dextrose Injection, USP
10% Dextrose Injection, USP (concentrated dextrose in water) is a sterile, nonpyrogenic, hypertonic solution of Dextrose, USP in water for injection for intravenous administration after appropriate admixture or dilution. 10% Dextrose Injection, USP is provided as a 500 mL volume in a 1000 mL partial-fill container. The container is designed to facilitate admixture or dilution. The solution contains no bacteriostat, antimicrobial agent or added buffer and is intended only for use as a single-dose injection following admixture or dilution.
The flexible plastic container is fabricated from a specially formulated polyvinyl chloride. Water can permeate from inside the container into the overwrap but not in amounts sufficient to affect the solution significantly. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. Exposure to temperatures above 25?/77? during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period.
Dextrose Injection, USP is a parenteral fluid and nutrient replenisher. Dextrose Injection, USP is chemically designated D-glucose monohydrate (C6H12O6 H2O), a hexose sugar freely soluble in water. It has the following structural formula:
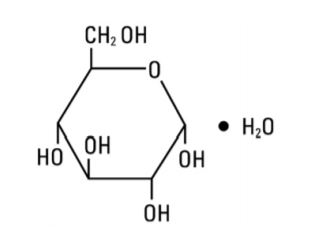
Water for Injection, USP is chemically designated H2O.
Clinical Pharmacology
When administered intravenously, solutions containing carbohydrate in the form of dextrose restore blood glucose levels and provide calories. Carbohydrate in the form of dextrose may aid in minimizing liver glycogen depletion and exerts a protein sparing action. Dextrose Injection, USP undergoes oxidation to carbon dioxide and water.
Water is an essential constituent of all body tissues and accounts for approximately 70% of total body weight. Average normal adult daily requirement ranges from two to three liters (1.0 to 1.5 liters each for insensible water loss by perspiration and urine production, respectively).
Water balance is maintained by various regulatory mechanisms. Water distribution depends primarily on the concentration of electrolytes in the body compartments, and sodium (Na+) plays a major role in maintaining physiologic equilibrium.
Indications and Usage
10% Dextrose Injection, USP (concentrated dextrose in water) in a partial-fill container is indicated for admixture with amino acids or dilution with other compatible I.V. fluids to provide a 5% final dextrose concentration for intravenous infusion in patients whose condition requires parenteral nutrition.
Contraindications
A concentrated dextrose solution should not be used when intracranial or intraspinal hemorrhage is present nor in the presence of delirium tremens if the patient is already dehydrated.
Dextrose Injection, USP without electrolytes should not be administered simultaneously with blood through the same infusion set because of the possibility that pseudoagglutination of red cells may occur.
Warnings
Concentrated dextrose in water should be administered only after suitable dilution. Hypertonic dextrose solutions should be given slowly. Significant hyperglycemia and possible hyperosmolar syndrome may result from too rapid administration. The physician should be aware of the symptoms of hyperosmolar syndrome, such as mental confusion and loss of consciousness, especially in patients with chronic uremia and those with known carbohydrate intolerance.
The intravenous administration of this solution can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema.
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
For Peripheral Vein Administration
Hypertonic dextrose solutions (above 5% concentration) should be given slowly, preferably through a small bore needle into a large vein, to minimize venous irritation.
For Central Venous Administration
Concentrated dextrose should be administered via central vein after appropriate admixture or dilution when required.
Precautions
Electrolyte deficits, particularly in serum potassium and phosphate, may occur during prolonged use of concentrated dextrose solutions. Blood electrolyte monitoring is essential, and fluid and electrolyte imbalances should be corrected. Essential vitamins and minerals also should be provided as needed.
Solutions containing dextrose should be used with caution in patients with known subclinical or overt diabetes mellitus.
Care should be exercised to insure that the needle (or catheter) is well within the lumen of the vein and that extravasation does not occur.
Concentrated dextrose solutionsshould not be administered subcutaneously or intramuscularly.
Do not administer unless solution is clear and container is undamaged. Discard unused portion.
Pregnancy Category C
Animal reproduction studies have not been conducted with dextrose. It is also not known whether dextrose can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Dextrose should be given to a pregnant woman only if clearly needed.
This product contains no more than 25 mcg/L of aluminum.
Adverse Reactions
Hyperosmolar syndrome, resulting from excessively rapid administration of concentrated dextrose may cause hypovolemia, dehydration, mental confusion and/or loss of consciousness. Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia. If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
Dosage and Administration
Concentrated Dextrose in Water is administered by slow intravenous infusion (a) after admixture with amino acid solutions or (b) after dilution with other compatible I.V. fluids. Dosage should be adjusted to meet the requirements of each individual patient.
The maximum rate at which dextrose can be infused without producing glycosuria is 0.5g/kg of body weight /hr. About 95% of the dextrose is retained when infused at a rate of 0.8g/kg/hr.
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations, and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
A list of nutritional admixture values is appended.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. See PRECAUTIONS.
Drug Interaction
Additives may be incompatible. Consult with pharmacist, if available. When introducing additives, use aseptic technique, mixthoroughly and do not store.
Some opacity of the plastic due to moisture absorption during sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
WARNING: Do not use flexible container in series connections.
VIAFLEX plastic container
The VIAFLEX plastic container is fabricated from a specially formulated polyvinyl chloride (PL 146 Plastic). The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g. di-2-ethylhexyl phthalate (DEHP), up to 5 parts per million. However, the safety of the plastic has been confirmed in tests in animals according to USP biological tests for plastic containers as well as tissue culture toxicity studies.
Why PVC?
Polyvinyl chloride (PVC) plastic is used to manufacture a huge number of articles for daily life, e.g. toys, building material such as flooring, cables, as well as medical products. PVC is the most widely used thermoplastic material in medical devices due to its:
Safety
Before medical devices can be used all the components must be fully understood from a toxicological point of view. Consequently all the materials used to make such components have to be thoroughly tested and assessed in the EU before being accepted. Experience based on all available knowledge from international environmental and healthcare authorities shows that PVC is safe. It is the best material existing today which optimises all performance and safety requirements at lowest cost.
Chemical stability
Material used in medical applications must be capable of accepting or conveying a variety of liquids without themselves undergoing any significant changes in composition or properties.
Biocompatibility
Whenever plastics are used in direct contact with the patients tissue or blood, a high degree of compatibility is essential between the tissue/blood and the material. The significance of this property increases with time over which plastic is in contact with the tissue or blood. PVC is characterised by high biocompatibility, and this can be increased further by appropriate surface modification.
Clarity and transparency
Because of its physical properties, products made from PVC can be formulated with excellent transparency to allow for continual monitoring of fluid flow. If colour-coded application is needed, virtually any colour can be created.
Flexibility, durability and dependability
Not only does PVC offer the flexibility necessary for applications such as blood bags and IV containers, but can also be relied upon for its strength and durability, even under changing temperatures and conditions.
Contains DEHP
Diethyl hexyl Phthalate, A chemical commonly used in PVC tubing; used to yield clarity and soft pliable tubing.
Product Characteristics

Packing Information
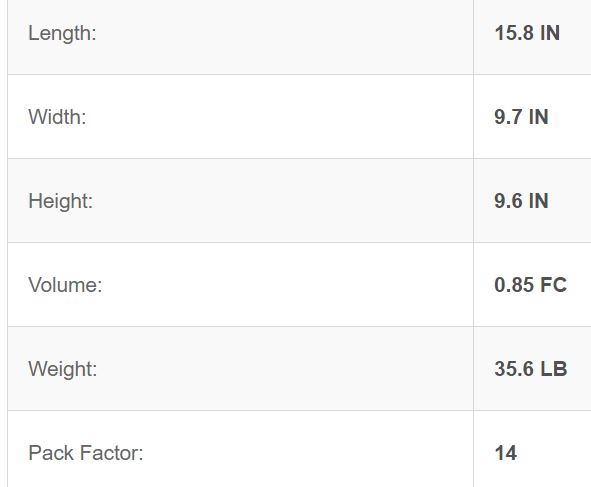

Baxter #2B0164X, Dextrose Injection, 10% USP, 1000 ml, Viaflex Plastic Container, 14/cs
$186.94 per CASE

Baxter #1A0694, DBD-MEDLINE CANNOT DISTRIBUTE-SOLUTION, N, 12/CS
$311.09 per CASE

Baxter #2B7731, REFRIDGERATE-BAG, CLINIMIX, 5/20 1 LT, 6/CS
$355.12 per CASE

Baxter #2B7726, SOLUTION, CLINIMIX(4.25%AMINO ACID/5%DEX), 6 EA/CS
$292.01 per CASE

