-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Baxter 2B1074X - Dextrose Solutions: Solution 5% Dextrose/0.45% Sodium Chloride, 1000 mL, 14 Per/Cs
Dextrose Solutions: Solution 5% Dextrose/0.45% Sodium Chloride, 1000 mL, 14 Per/Cs
Dextrose and Sodium Chloride Injection USP solutions are sterile, nonpyrogenic and contain no bacteriostatic or antimicrobial agents or added buffers. The composition, osmolarity and approximate pH of the individual solutions are shown below:
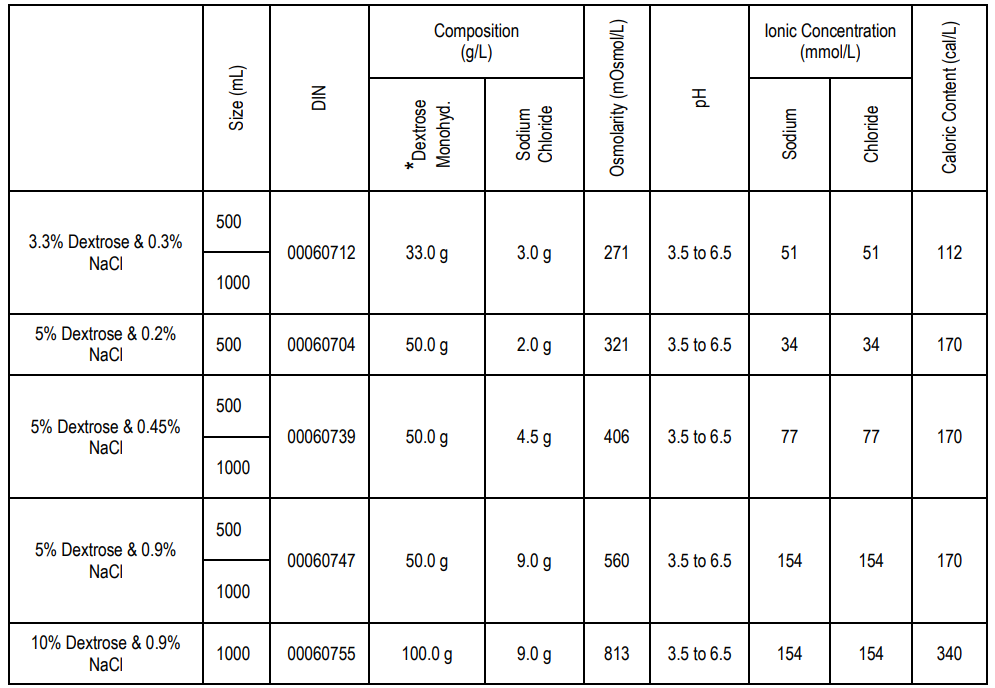
Water in a solution in the container can seep through the plastic wall, but in an insufficient amount to affect the solution. Before the product expires, a small number of chemical components present in the plastic and be leached into the solution in the container, such as up to 5 parts per million for di-2-ethylhexyl phthalate (DEHP). No safety issues found in the USP biological tests in animals as well as by tissue culture toxicity studies.
Baxter 5% Dextrose / 0.45% Sodium Chloride Clinical Use and Indications
Dextrose and Sodium Chloride Injection solutions are a source of water for hydration and provide electrolytes and calories. They are capable of inducing diuresis depending on the clinical conditions of the patient. Dextrose and Sodium Chloride Injection solutions are indicated as a supply of water or for administration of electrolytes or calories. Dextrose liquid is purified from corn and may contain fructose.
Baxter 5% Dextrose / 0.45% Sodium Chloride Contraindications
Dextrose and Sodium Chloride Injection is contraindicated when:
- Treating patients who are hypersensitive to this drug or the ingredients in the formulation and components in the container.
- Treating patients who have known allergies to corn products.
- Treating patients who have clinically significant hyperglycemia.
VIAFLEX plastic container is made from formulated polyvinyl chloride.
- Manufacturer: Baxter 2B1074X
- Country of Origin: USA
- Application: Caloric Agent
- Container Type: Flexible Bag
- Dosage Form: IV Solution
- Generic Drug Name: Dextrose / Sodium Chloride
- Strength: 5% - 0.45%
- Type: Intravenous
- Volume: 1,000mL
- Latex Free Indicator: Not Made with Natural Rubber Latex

Baxter #2B1064, 5% Dextrose And 0.9% Sodium Chloride Injection USP in VIAFLEX Plastic Container, 1000 mL, 24 Per/CS
$344.90 per CASE

Baxter #2B2544X, SOLUTION, PLASMA-LYTE A INJ PH 7.4, 1000ML, 14 PER/CS
$398.56 per CASE

Baxter #2B2324X, SOLUTION, RINGER'S, LACTD, INJECTION, 1000ML, 14 PER/CS
$149.50 per CASE

