-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Baxter 6N8220 - KIT, EXTENSION SET, CATHETER, TUBING, DEVICE, 50 EA/CS
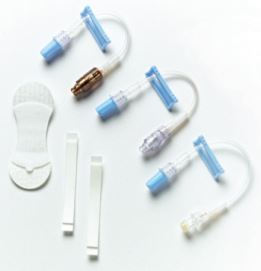
Baxter Catheter Stabilization Device Extension Set Kits
V-LINK Device with VITALSHIELD Protective Coating Non-DEHP Catheter Extension Set Kit, Standard Bore Tubing, 1 luer activated device with silver for IV Access, Medium VITAL HOLD Catheter Stabilization Device, foam adhesive strips. Holds tubing and lines 3.0 mm to 8.0 mm in diameter.
VITAL HOLD Catheter Stabilization Device
Promote Consistent, Convenient, Cost Effective Care
Enhance patient care with comfortable, reliable securement.
- Catheter securement devices are recommended to "decrease the risk for phlebitis, catheter migration and dislodgement, and may be advantageous in preventing CRBSIs" in the 2011 CDC Guidelines for the Prevention of Intravascular Catheter-Related Infections.
- INS Standards of Practice advocate catheter stabilization devices as "the preferred alternative to tape or sutures when feasible."
- Use of catheter stabilization devices reduces risk of needlestick injuries and associated complications.
- Baxter catheter stabilization devices have been demonstrated to reduce the rate of unscheduled restarts by up to 62% when compared to tape4 , which decreases facility costs.
- VITAL HOLD Products offer familiar designs that nursing professionals can consistently apply.
- VITAL HOLD Devices are comfortable for patients to wear due to their soft, non-rigid designs.
Reduce Inventory
Reduce steps and costs for you and your facility with kits
- VITAL HOLD Kits contain key components necessary for safe IV catheter securement in compact packages.
- Each kit reduces the need for additional components, like tape.
- Kits reduce SKUs, decrease inventories and help nurses reduce steps when collecting supplies.
VITAL HOLD Catheter Stabilization Kits are available with three extension set options for IV Access:
- The INTERLINK System
- The CLEARLINK System
- V-LINK Luer-Activated Device with VITALSHIELD protective coating
V LINK Luer Activated Device with VITALSHIELD Protective Coating
The first antimicrobial IV connector
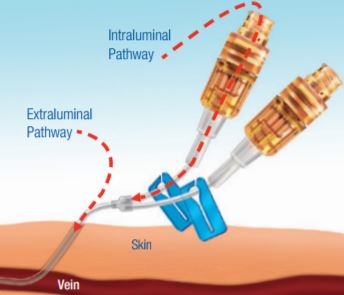
Technique and technology are needed to fight pathogens
- Every day, you do all you can to prevent microbial contamination - Proper aseptic technique at the IV insertion site can reduce extraluminal contamination (outside the catheter walls)
- Study results concluded that after specific preventive measures have been taken, 60% of catheter-related bloodstream infections (CR-BSIs) are likely to originate from the catheter fluid path1 Technology to help protect the intraluminal pathway (the fluid path) is also needed to further reduce the risk of CR-BSIs
- Fights pathogen contamination and microbial growth in the fluid path
- Consistently high efficacy across multiple bacterial strains
- Demonstrated durability and efficacy over 96 hours
A well-known design
Proprietary VitalShield silver antimicrobial coatingEmpowering you to fight an ever-present danger
The VitalShield protective coating is a uniquely designed formulation of silver nanoparticles that helps prevent microbial contamination and growth of pathogens within the V-Link device. *The V-Link device is contraindicated for individuals with hypersensitivity to silver or silver components. |
|