-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

BD 220526 - Universal Viral Transport Kit: 1 mL vial with a flexible minitip flocked swab, 50/PK
BD 220526 Universal Viral Transport Kit: 1 mL vial with a flexible minitip flocked swab
BD Universal Viral Transport (UVT) System includes a single formulation that is room temperature stable. BD UVT can sustain viability (and infectivity) of a plurality of organisms that include clinically important viruses, chlamydiae, mycoplasmas and ureaplasmas. The formulation of BD UVT medium is specially designed to include protein for stabilization, antibiotics to minimize bacterial and fungal contamination and a buffer to maintain a neutral pH. As a result, viruses and clamydiae can be preserved for long-term storage when frozen.
The unique BD UVT formulation offers flexible transport temperature in a single formulation eliminating the potential for false negative results and sample rejection due to improper transport temperature or an incorrect formula choice.
- Room temperature and refrigerated viability in ONE formulation
- Compatible transport medium for the BD Directigen EZ viral tests
- Designed with safety and convenience to meet your viral testing needs
BD Universal Viral Transport (UVT) System is a room temperature stable, universal medium formulation for the collection and transport of clinical specimens containing viruses, chlamydiae, mycoplasmas and ureaplasma from the collection site to the testing laboratory. The formulation of BD UVT medium is specially designed to include protein for stabilisation, antibiotics to minimise bacterial and fungal contamination and a buffer to maintain a neutral pH.
The design of the BD Universal Viral Transport System increases laboratory efficiency and safety with the following features:
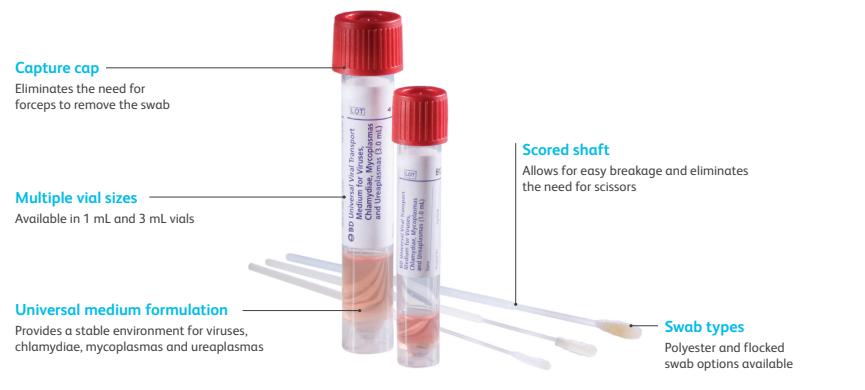
Unique Formulation of BD Universal Viral Transport
Efficient Design of BD Universal Viral Transport
Compatibility
|  |
Device Characteristics of BD 220244 Universal Viral Transport Kit: 1 mL vial with a flexible minitip flocked swab
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |

BD #220526, Universal Viral Transport Kit: 1 mL vial with a flexible minitip flocked swab, 50/PK
$351.24 per PACK

BD #220531, Universal Viral Transport Kit: 3 mL vial with a flexible minitip flocked swab, 50/PK
Call for Pricing

BD #220222, Universal Viral Transport Combo Kit: 3 mL vial with 1 regular and 1 minitip wire/plastic swab, 50/PK
$320.00 per PACK

BD #220529, BD Universal Viral Transport System, KIT, COLLECTION, 3ML, MINITIP FLOCKED SWAB, 50 PER/CS
$539.04 per CASE


