-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Cook Medical G32724 - DEVICE, NTRAP, ENTRPMNT, EXTRCTN, NTP-028145, EACH
NTrap Stone Entrapment and Extraction Device
The NTrap Stone Entrapment and Extraction Device is used to remove calculi and other foreign bodies in the urinary tract, and to minimize stone migration during laser, ultrasonic, electrohydraulic, or pneumatic lithotripsy.
Set contains (set components may vary)
- Stone Extractor
- Unidex Handle
| Order Number | Reference Part Number | Fr | Length (cm) | Basket Configuration |
| G32724 | NTP-028145 | 2.8 | 145 | 7 mm umbrella design |
Features and Benefits
- Basket design: The intricately woven, multiwire basket prevents the migration of fragments that are as small as 1.5 mm in diameter.
- Nitinol construction: The resilient shape-memory characteristics of the nitinol wire allow the basket to retain its shape after deployment.
- Flexible sheath: The 145 cm long sheath allows efficient scope exchange.
- Removable handle: While the basket is deployed, the handle can be detached so that the scope can be withdrawn. Removing the scope from the working channel of the access sheath allows increased irrigation.
Intended Use
Used as an endoscopic entrapment and extraction device for calculi and other foreign bodies in the urinary tract, and to minimize stone migration during laser, ultrasonic, electrohydraulic or pneumatic lithotripsy.
How To Use
- Using direct vision or fluoroscopic control, advance the NTrap sheath beyond the stone or foreign object. Deploy the basket by advancing the handle while holding the sheath in position.
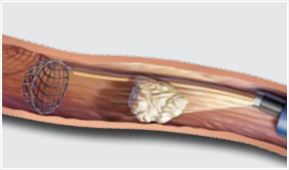
- Use an intracorporeal lithotripter to fragment the stone. To avoid damaging the NTrap device when you use laser or electrohydraulic lithotripsy, maintain a gap of at least 1 cm between the stone and the NTrap device. Avoid firing the laser directly on the NTrap.

- Use the basket to gather stone fragments and prevent fragments as small as 1.5 mm from migrating.

- To complete the procedure, close the basket by advancing the sheath while holding the handle in position. Gently advance the basket to disengage stones that are too large for extraction.

Precautions
- Users should be familiar with and experienced in urological endoscopic surgery.
- Assess calculi or other foreign bodies prior to instrument deployment to ensure that object is not too large to be removed through the anatomy.
- If resistance is encountered while attempting to remove calculi or other foreign bodies, release the object. Do not exert excessive force on the device.
- Due to the asymmetric nature of the NTRAP, do not torque or rotate the device in vivo.
- When used in conjunction with holmium: YAG laser, do not fire the laser directly upon the device.
- This device is conductive. Avoid contact with any electrified instrument.
- Enclose device in sheath before removing from tray/holder.
Potential Adverse Events
The following complications are possible with the use of stone extraction devices.
- Tissue perforation
- Tissue evulsion
- Edema
- Hemorrhage
Instructions for Use
- Using aseptic technique, remove the NTRAP from its outer package and place into the sterile field. The NTRAP operating handle is attached to facilitate opening or closing.
- Before insertion, retract the NTRAP basket into the sheath.
- Using direct vision or fluoroscopic control, advance the NTRAP sheath beyond the stone or foreign object prior to deployment.
- Deploy the NTRAP by advancing the handle forward while holding the sheath in position. The device may now be used to minimize stone migration during laser, ultrasonic, electrohydraulic or pneumatic lithotripsy.
NOTE: When using laser or electrohydraulic lithotripsy, a gap of at least 1 cm should be maintained between the stone and the NTRAP to prevent device damage.
NOTE: The NTRAP, in the open position, may be used as an extraction device to sweep the ureter, collecting stone fragments into the bladder. The operating handle may be detached to facilitate scope removal. - Upon completion of the procedure, close the NTRAP basket by pushing the sheath forward while holding the operating handle in position.
- It may be necessary to gently advance the basket to release any remaining stone fragments prior to closing.
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |
How Supplied
Supplied sterilized by ethylene oxide gas in peel-open packages. Intended for one-time use. Sterile if package is unopened or undamaged. Do not use the product if there is doubt as to whether the product is sterile. Store in a dark, dry, cool place. Avoid extended exposure to light. Upon removal from the package, inspect the product to ensure no damage has occurred.
CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician (or a properly licensed practitioner).


