-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Ethicon 4150 - BioPatch Protective Disk with CHG 1' 4mm 10/BX, 4 BX/CS
Ethicon 4150 BIOPATCH Protective Disk with CHG
BioPatch Antimicrobial Dressing with Chlorhexidine Gluconate is a hydrophillic polyurethane absorptive foam with Chlorhexidine Gluconate (CHG) which inhibits bacterial growth under the dressing. The dressing is intended to be used to absorb exudate, cover a wound caused by vascular and nonvascular percutaneous medical devices during surgery, as well as reduce local infection and colonization of microorganisms.
The BIOPATCH Protective Disk with CHG is the evidence-based dressing choice for reducing local infections, catheter-related blood stream infections (CRBSI) and skin colonization of microorganisms commonly associated with CRBSI in patients with central venous andarterial catheters.
Protect all lines. Protect all lives
It is estimated that over 54,000 people get a catheter-related bloodstream infection (CRBSI) every single year in the UK.
BIOPATCH Protective Disk with CHG is the only dressing that meets all the following criteria:
Intended to reduce local infections, catheter related bloodstream infections (CRBSI), and skin colonization of microorganisms commonly related to CRBSI, in patients with central venous or arterial catheters.
- Is constructed from polyurethane foam allowing quick absorption of fluid decreasing the likelihood of skin maceration.
- Is designed to deliver chlorhexidine gluconate a full 360 degree around the catheter insertion site providing optimized coverage and protection.
- BIOPATCH has been shown to reduce catheter-related bloodstream infections (CRBSIs) by up to 60% in central venous and arterial catheters, even when infection rates are low.
Even after treatment with topical antimicrobials, resident bacteria from the patients own skin quickly re-colonize.
For use with both vascular and nonvascular percutaneous devices

The Problem
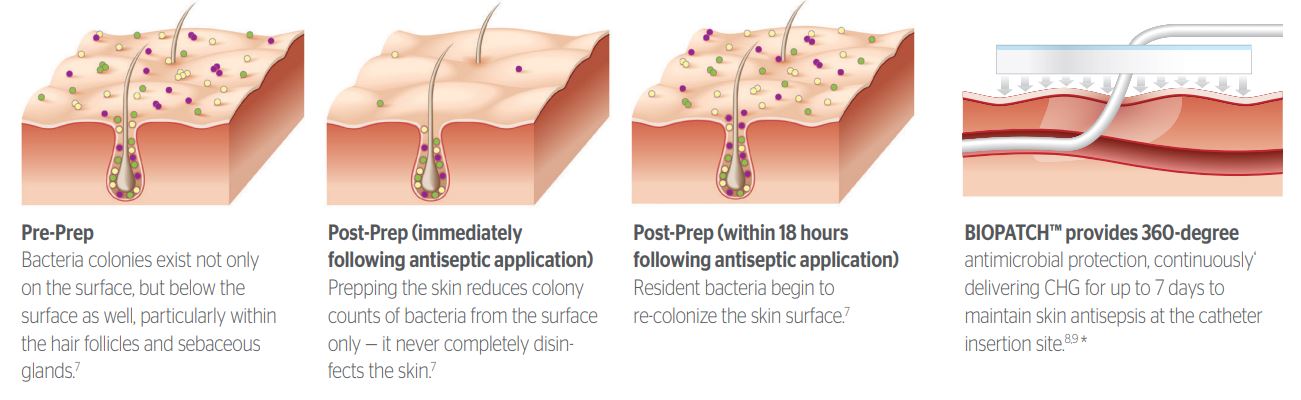
Prepping the skin is not enough: 60% of CRBSI originate from the patient's own skin. Without continual suppression, bacteria on the skin surface can REPOPULATE and migrate into the bloodstream, elevating the risk of CRBSI.
Within hours of thorough antiseptic application, resident bacteria quickly re-colonize the skin surface.
1. Pre-Prep:
Bacteria colonies exist not only on the surface, but below the surface as well, particularly within the hair follicles and sebaceous glands.
2. Post-Prep:
(Immediately following antiseptic application)
Prepping the skin reduces colony counts of bacteria from the surface only - it never completely disinfects the skin.
3. Post-Prep:
(within 1-2 days following antiseptic application)
Resident bacteria begin to re-colonize on the skin surface.
The Solution
BIOPATCH Protective Disk with CHG is the Solution to Reducing Risk of Catheter-Related BSIs.
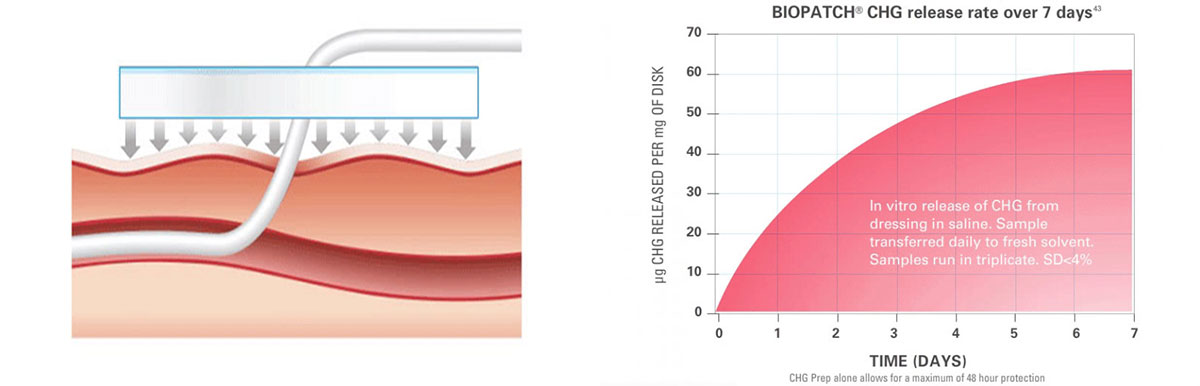
CHG Delivery
Through its proprietary delivery technology, BIOPATCHprovides proven sustained antimicrobial action over 7 days.
Continuous release of CHG provides 360 protection around the insertion site for 7 days for ongoing antisepsis between dressing changes.
CHG Accumulation
BIOPATCH delivers a clinically relevant dose of CHG.