-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

HLIC HLEF84722_DISC - Extension set w/filter, 10" standard set, .22 Micron Filter, Side clamp, Rotating Male Luer Lock, 50 Per/Cs (Expired. Not for use on humans)
Standard Bore Extension Set w/0.22 Micron Filter, Female Luer Connection, Slide Clamp, Distal Spin Connector. Length: 10 in. (25.4 cm)
Extend your IV therapy without overextending yourself!
Features
- Female Luer Connection.
- Slide Clamp.
- Distal Spin Connector.
- Length: 10 in. (25.4 cm).
Slide clamp
Slide clamp used to regulate the flow of IV solution. The slide clamp has a graduated opening through which the IV tubing passes. Pushing the tube into the narrow end of the opening constricts it and reduces the flowrate of the IV solution. Sliding the wide end of the opening over the tube increases the flow rate.
Filter Material
The filter material of HLIC filter sets is polyethersulfone (PES). The size of the membrane pore are 0.22 micron. The multiple layer, three-dimensional filter, significantly increases the capability of entrapping microparticles and reduces drug absorption rate; and there is no membrane shedding. This prevents phlebitis, vascular discoloration or hardening, local embolisms, scleroma, limb numbness, granuloma, etc. thus ensuring a safe, clinical infusion.
PES (Polyethersulfone) Membrane
These hydrophilic, low protein binding, PES membrane filters are ideal for tissue culture media sterilization, life science and microbiology fluid applications, clinical, and general filtration. Microporous PES membranes are produced from a high-temperature, acid and base resistant, polyethersulfone polymer in a proprietary, quality-assured manufacturing process.
The high level of quality standards applied in each step of production provide consistent products with pore uniformity for predictable flow rates, particle retention, and purification values. Inherent, uniform porosity and controlled pore size allow these PES filtration membranes to efficiently remove particulates from solutions during general filtration and its low protein and drug binding characteristics maximize recovery of critical drugs used in IV therapy, chemotherapy, and open-heart surgery. The strength and durability offered by our PES membranes is suited for aggressive handling and use with automated equipment.

0.22 Micron Filter
Membrane filters with pore-size rating of 0.22 micron were tested for it's ability to recover Pseudomonas diminuta ATCC 19146 (P. diminuta), the organism typically used in bacterial retention testing of sterilizing-grade membrane filters. 0.22 micron filters have ability to retain higher levels of bacteria.
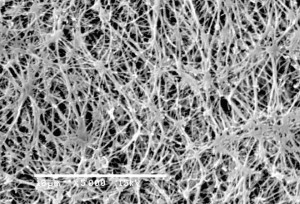
0.22 micron filter used to sterilize fluid passed through it. So, for the purpose of sterilization, 0.22 micron filters are indistinguishable. The real measure of filters ability to sterilize fluid is passing the test described in ASTM F838-05, Standard Test Method for Determining Bacterial Retention of Membrane Filters Utilized for Liquid Filtration. Basically, if the filter can retain a minimum of 1 x 107 colony forming units (cfu) per cm2 of a challenge bacteria (usually B.diminuta5), then the filter is suitable to use for sterilization.
Female Luer
What is a "Luer"?
The Luer taper is a standardized system of small-scale fluid fittings used for making leak-free connections between a male-taper fitting and its mating female part on medical and laboratory instruments, including hypodermic syringe tips and needles or stopcocks and needles.
DEHP Free
Infusion Therapy Standards of Practice advise to usee administration sets free of di-ethylhexyl-phthalate (DEHP) to administer lipid-based infusates, such as IVFE or TNA. DEHP is lipophilic and is extracted into the lipid solution with commonly used polyvinyl chloride administration sets and containers. DEHP is considered a toxin, and studies have demonstrated increased DEHP levels in lipid solutions, which is especially a risk with neonatal, pediatric, and long-term home care patients (42).



