-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Integra Lifesciences INS8420 - TraumaCath Ventricular Catheter Set, EACH
Integra Lifesciences INS8420 TraumaCath Ventricular Catheter Set
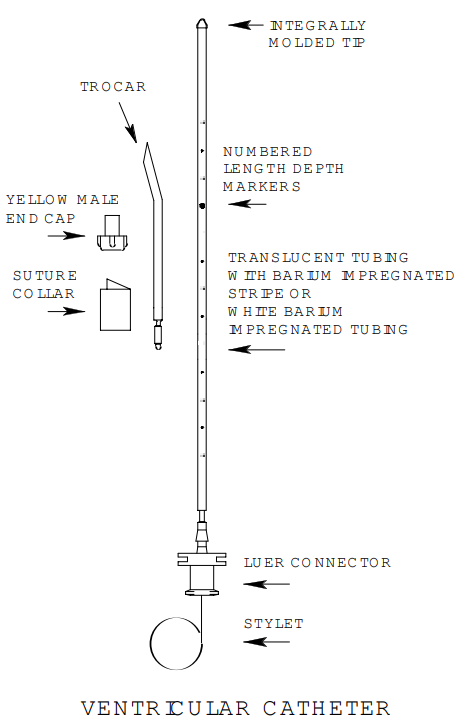
The Ventricular Catheters are designed for diverting fluid from the ventricles through a series of drainage holes near the catheter tip. The catheters can be inserted into the ventricular cavity with the stainless steel stylet. A trocar is supplied with the catheters to facilitate subcutaneous tunneling away from the burr hole. The external portion of the catheter may be secured to the scalp by the suture collar.
All external ventricular catheters have markings from 3 to 15 cm 1.5 mm with numbers located at odd markings and dots located at even markings. All catheters have a radiopaque tip.
The luer connector supplied with each ventricular catheter set will connect the catheter to any Integra NeuroSciences External Drainage System. The INS8220 and INS-0001 catheters are barium impregnated and the INS-4000, INS-4500 and INS-8420 are barium striped for radiopacity.
Integra Lifesciences INS8420 TraumaCath Ventricular Catheter Features and Benefits
- Fabricated from translucent silicone elastomer tubing with a bariumsulfate impregnated stripe or white barium-impregnated tubing.
- Kink and compression resistant tubing.
- Stainless steel stylet.
- Luer connector and yellow luer male end cap.
- Numbered depth markers.
- Silicone suture collar.
- Stainless steel trocar.
- Large bore tubing and enlarged inlet holes to promote drainage.
- Smooth tantalum impregnated tip integrally molded to catheter.
Indications of Integra Lifesciences INS8420 TraumaCath Ventricular Catheter
External ventricular drainage catheters are indicated for drainage and monitoring of CSF from the lateral ventricles of the brain. The catheters may be used to reduce intracranial pressure (ICP), to monitor ICP, to monitor CSF and in the management of hydrocephalic shunt infections. External lumbar drainage catheters are indicated for drainage and monitoring of CSF from the lumbar subarachnoid space.
Contraindications for Integra Lifesciences INS8420 TraumaCath Ventricular Catheter
These devices are not designed, sold or intended for use except as indicated. Lumbar drainage and/or lumbar pressure monitoring should not be used in the presence of: non-communicating hydrocephalus; a large intracranial mass, tumor or hematoma; or in patients who have demonstrated a blockage of cerebrospinal fluid pathways due to trauma, tumor, hematoma or other large intracranial mass.
Lumbar catheters are contraindicated in cases of spinal abnormalities that would prevent free insertion of the lumbar catheter. Lumbar catheters are contraindicated in infants where the lower end of the spinal cord has not yet migrated to its cephalad L1-2 position. In view of the marked narrowing of the lumbosacral canal in achondroplastic patients, a lumbar catheter in the subarachnoid space is contraindicated.
Integra Lifesciences INS8420 TraumaCath Ventricular Catheter Instructions for Use
- Introduce and position the ventricular catheter into the ventricle. Placement of these catheters may be accomplished through a variety of surgical techniques; therefore, the surgeon is best advised to use the method which his/her own practice and training dictate to be best for the patient.
- Verify placement of catheter in ventricle. Remove stylet with luer connector for use in step #4.
- Tunnel subcutaneously with the trocar, using the surgical technique of the surgeon.
- Slide barbed luer connector from stylet into catheter and press firmly.
- Check for free flow of CSF and then cap the catheter. Care should be taken not to cut or tear the tubing when placing ligatures.
- Slide the suture collar over the catheter to the desired position. Suture the collar to the scalp.
- Follow the instructions included with the drainage system for recommended drainage procedures.
Device Characteristics of Integra Lifesciences INS8420 TraumaCath Ventricular Catheter
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | Yes |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |


